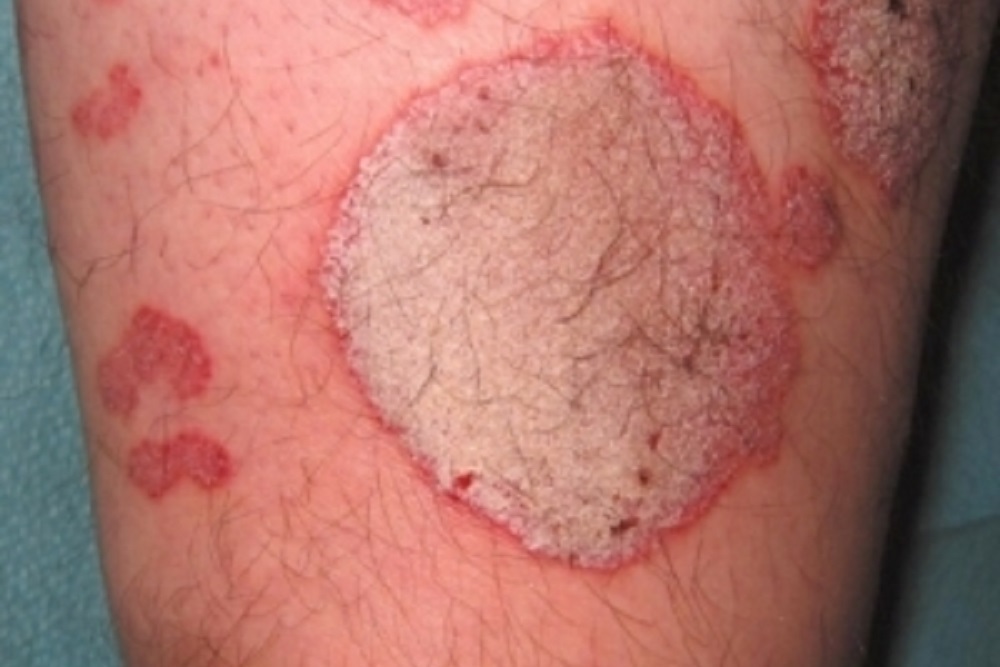
Novartis, an immunology and dermatology company, recently reported positive five-year efficacy and safety results for Cosentyx after completing a Phase III long-term extension study in patients with moderate-to-severe plaque psoriasis.
The company noted that data from the study will be presented later in 2017 at a key medical congress. The study was created to analyze efficacy and safety of the treatment for psoriasis, which is a common, non-contagious, auto-immune disease affecting more than 125 million people across the globe.
Cosentyx works by specifically inhibiting the IL-17A cytokine that plays a role in driving inflammation. The study showed promising results after four years; data presented at EADV 2016 showed Cosentyx to almost clear or completely clear skin in most patients.
"Cosentyx has consistently demonstrated sustained efficacy and safety providing psoriasis patients a new standard of long-term care," Vas Narasimhan, global head of drug development and chief medical officer of Novartis, said in a statement. "With the first data from a pivotal trial with five years of follow up, Cosentyx continues to demonstrate it can provide what psoriasis patients want, a life with clear skin."







 Alerts Sign-up
Alerts Sign-up