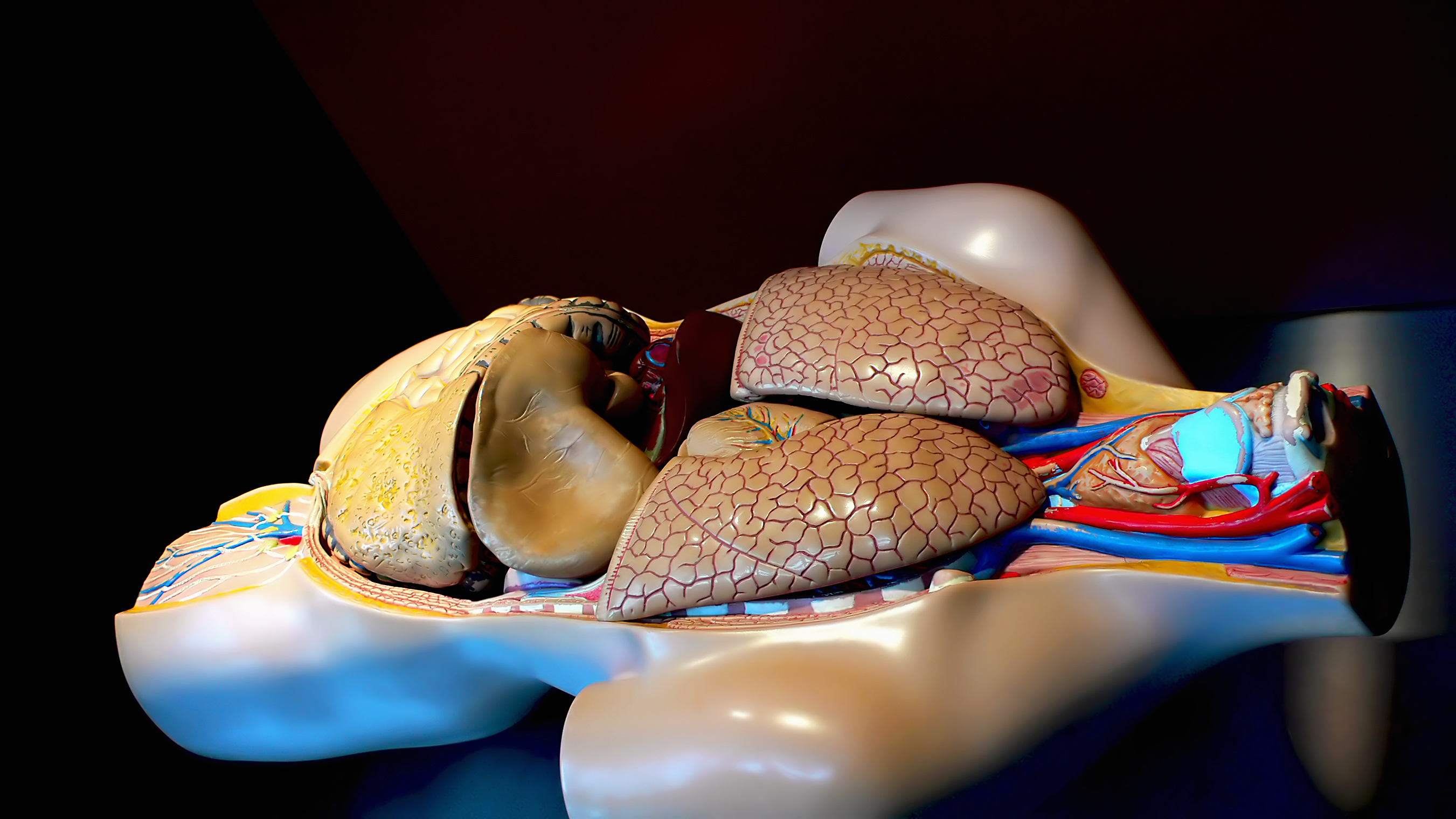
Albireo announced Thursday that it is beginning a phase II trial with the company’s lead compound for cholestatic liver disease and NASH, A4250.
The study will determine the safety and efficiency of A4250 in patients with cholestatic puritus and primary biliary cirrhosis (PBC). The trial will be led by Hanns-Ulrich Marschall, an expert in the field of liver diseases and bile acid alteration, and be conducted at the Sahlgrenska Academy in Göteborg, Sweden.
“The safety and efficacy of A4250 in both preclinical and phase I studies are encouraging and strongly support development of A4250 in cholestatic liver diseases,” Albireo's Chief Medical Officer Hans Graffner said.
A4250 inhibits the ilieal sodium dependent bile acid transporter (IBAT) in the stomach area. A4250 reduces the reabsorption of bile acids, thus decreasing levels of toxic bile acids in patients with cholestatic liver disease. It works locally, which reduces the risk of side effects.
“There is a real need for novel therapies in this area and A4250 clearly has the potential as a novel hepatoprotective drug that may help improve liver function in patients with a variety of chronic liver diseases,” Marschall said. “In phase I studies, A4250 has shown to be safe and shown clear effects on a number of key pharmacodynamic markers, including significant, dose-dependent lowering of serum bile acids. Serum bile acids are commonly increased in cholestatic liver diseases. I am excited about the possibility to test A4250 in patients with PBC having severe pruritus/itching”








 Alerts Sign-up
Alerts Sign-up