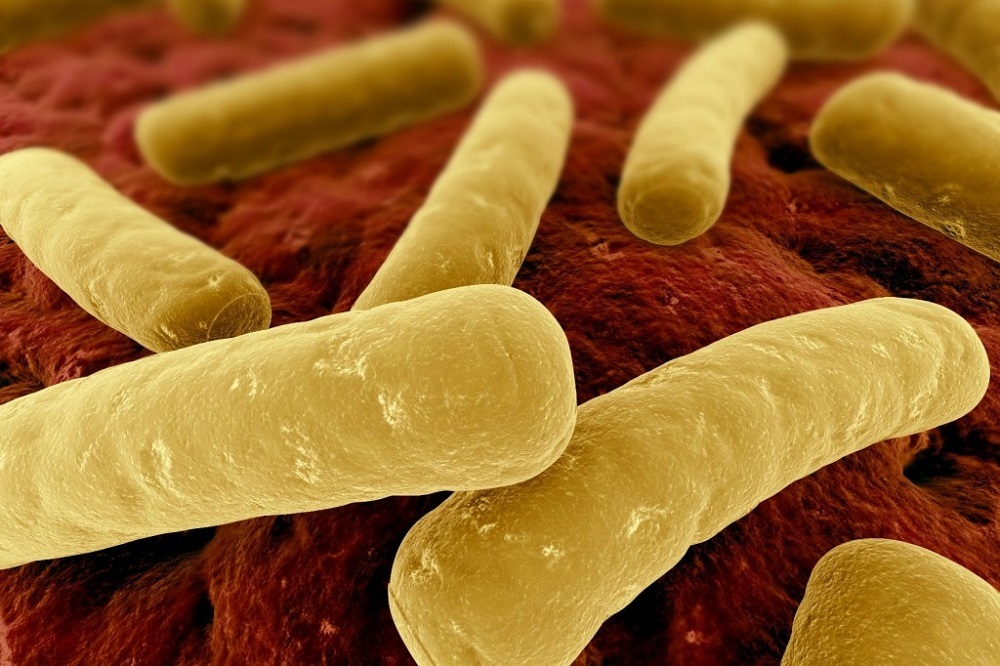
Pfizer Inc. recently shared positive data from its Phase 2 study examining Clostridium difficile (C. difficile) vaccine candidate, PF-06425090, a product created to help prevent C. difficile infection (CDI), which can include life-threatening diarrhea and pseudomembranous colitis.
The drug induces a functional antibody response in an attempt to neutralize the C. Difficile’s two main disease-causing toxins.
“Despite improved infection control measures, C. difficile disease continues to rise, further augmenting an already urgent public health threat with particular negative impact on older adults,” Dr. Kathrin Jansen, senior vice president and head of Vaccine Research and Development for Pfizer Inc., said in a statement. “We are very encouraged by these interim immunogenicity and safety results demonstrating robust increases in vaccine-elicited neutralizing antibodies to both toxins, that we believe could provide protection against C. difficile disease.”
Pfizer will move forward with a Phase 3 study this year of the vaccine, which was granted Fast Track designation in 2014 by the U.S. Food and Drug Administration (FDA).
Clostridium difficile, a spore-forming pathogen, often targets people with altered gut microbial flora. The pathogen releases toxins that lead to diarrhea, pseudomembranous colitis, toxic megacolon, intestinal perforation, or, in the most severe cases, death.







 Alerts Sign-up
Alerts Sign-up