American Pharmacists Association issued the following announcement on Dec. 18.
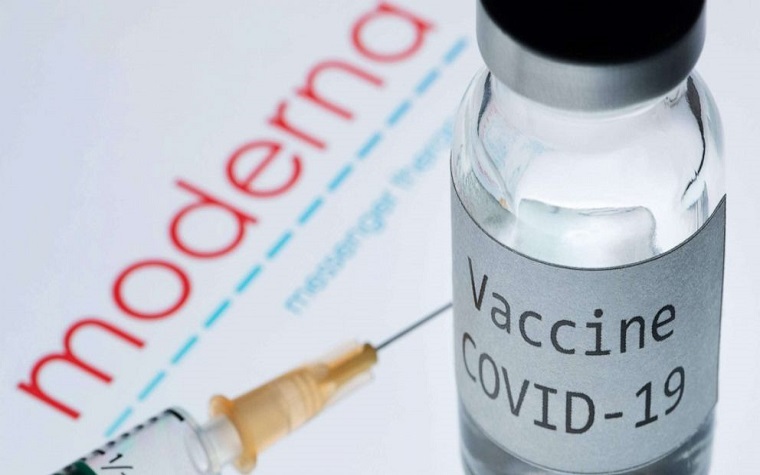
Statement from Scott J. Knoer, MS, PharmD, FASHP, executive vice president and CEO of the American Pharmacists Association, upon the Food and Drug Administration's (FDA) decision to authorize emergency use of Moderna's COVID-19 vaccine:
"With the FDA's action today, pharmacists are again prepared to step forward and serve as essential health care providers in the historic effort to control and end this pandemic.
"Throughout this past week, as the Pfizer-BioNTech vaccine found its way into institutions and communities across the country, trained and trusted pharmacists, student pharmacists and pharmacy technicians delivered the vaccine to thousands of front-line health care providers and residents of long-term care facilities. I expect the same to happen next week, when nearly 6 million doses are shipped to more than 3,000 locations across the country.
"We sincerely hope that the FDA's actions over the past week mark the beginning of the end of the pandemic. We are confident in the care that the FDA has taken to ensure that the Moderna vaccine is safe and effective. Likewise, the American public should also have great confidence in the vaccine as an essential and effective component to the national effort, along with mask wearing and social distancing."
About the American Pharmacists Association
The American Pharmacists Association is the largest association of pharmacists in the United States advancing the entire pharmacy profession. Our expert staff and strong volunteer leadership, including many experienced pharmacists, allow us to deliver vital leadership to help pharmacists, pharmaceutical scientists, student pharmacists and pharmacy technicians find success and satisfaction in their work, while advocating for changes that benefit them and their patients. For more information, please visit www.pharmacist.com.
Original source can be found here.












 Alerts Sign-up
Alerts Sign-up