Onzetra Xsail featured at American Headache Society conference
The medication was approved by the U.S. Food and Drug Administration (FDA) in January of this year and is available by prescription for treatment against migraine with or without an aura in adults.
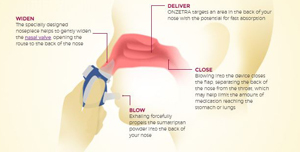
“We believe that Onzetra Xsail — which delivers a fast-acting dry powder dosage of a leading migraine treatment in the first Breath Powered® intranasal delivery system — addresses one of the greatest challenges faced by migraine sufferers: obtaining rapid relief with a known side effect profile,” Avanir CEO and President, Rohan Palekar said.
A total of six presentations were made and featured data from studies that focused on the safety of using the medicine and pharmacokinetic information.
The product delivers medication through the nasal cavity and contains sumatriptan, which is a commonly prescribed treatment for migraines. When in a powder form, it is absorbed consistently through this method. The company states that sumatriptan has been used for approximately 20 years.
The medicine is not meant to be used to prevent migraines from occurring and it should only be used in cases where a migraine diagnosis has been made. Onzetra Xsail has not been established as a treatment for cluster headaches.








 Alerts Sign-up
Alerts Sign-up