Two high-cost arthritis medications — one the world’s best-selling drug — may soon make way for generic equivalents following preliminary committee approval, but the Food and Drug Administration (FDA) must still decide whether the products are fully interchangeable.
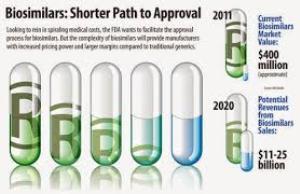
The primary drugs, Humira and Enbrel, are expensive biologics, created from living material rather than chemicals that address chronic conditions such as rheumatoid arthritis and plaque psoriasis. Patients would find immediate financial relief with generic versions, particularly seniors; Medicare spent $1.2 billion on Humira alone in 2014.
“They are very meaningful because this is a category that’s been growing year over year not only in price but in utilization as well,” Jeremy Schafer of Precision for Value, a health care economics consultancy, who presented on generics at an April pharmaceutical industry convention in San Francisco, said in a report in Roll Call. “More patients have been using them and the price of many of the products has been going up.”
The onus remains on the FDA to reassure patients of generics’ viability, however, pending its final seal of approval. Seth Ginsburg, co-founder of arthritis support organization CreakyJoints, stated that patients could be reluctant to substitute medications when using the original.
“Patients are indeed concerned about switching because there [haven’t] been substantial or sufficient studies or evidence about what happens when you switch,” Ginsburg said.
Additionally, legislators have expressed uncertainty and frustration at the lengthy approval process. The alternative products raise many legal issues as well as health reservations; FDA officials hope to publish interchangeability guidance this year.










 Alerts Sign-up
Alerts Sign-up