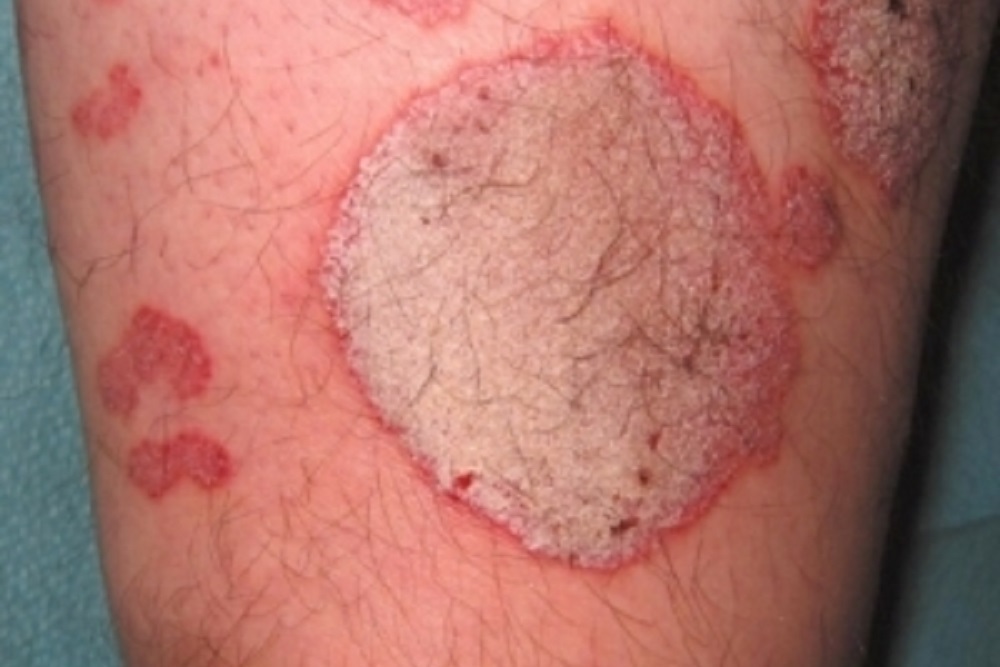
Janssen noted that the data showed that patients saw major improvements in skin clearance when taking the company’s product compared to a placebo. Additionally, those patients on the drug had greater improvements than those taking the anti-tumor necrosis factor-alpha treatment Humira (adalimumab).
“The majority of patients treated with guselkumab achieved high levels of skin improvement (more than 80 percent IGA 0/1 and nearly 70 percent PASI 90) at week 16, while this was rarely seen in patients receiving placebo (less than 10 percent); a difference that was highly significant. Higher rates in efficacy in major secondary endpoints comparing guselkumab with adalimumab were also demonstrated and significant,” Dr. Kristian Reich, VOYAGE 2 study investigator, said. “These findings are consistent with the previously presented Phase 3 VOYAGE 1 study results and further demonstrate the important role of selectively targeting IL-23 in an immune-mediated disease like plaque psoriasis.”
The company shared results at the 2017 American Academy of Dermatology Annual Meeting in Orlando, Florida.








 Alerts Sign-up
Alerts Sign-up