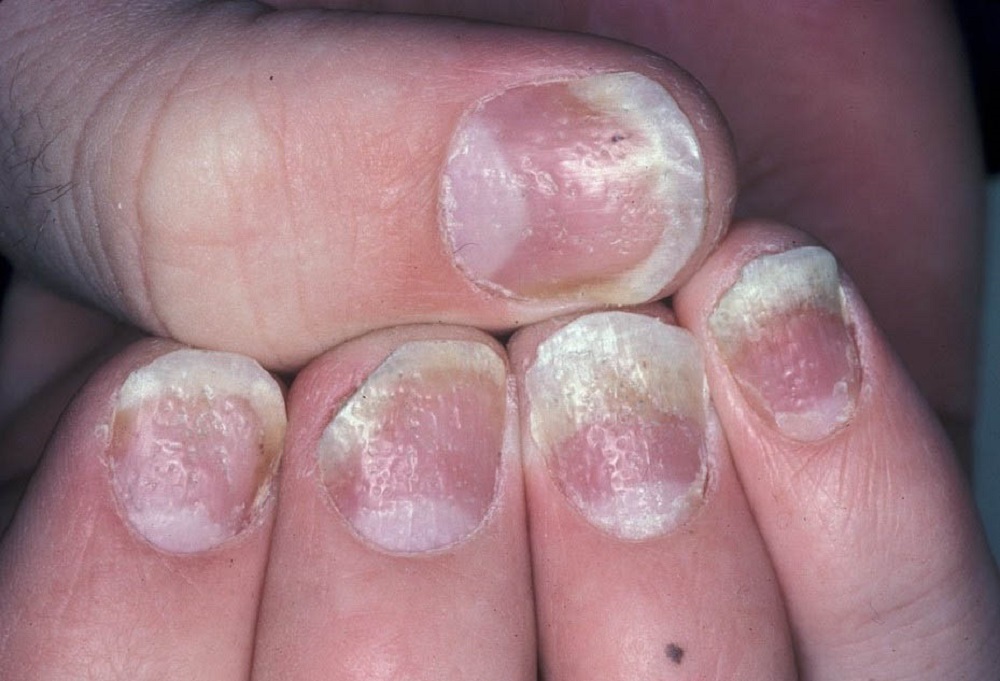
According to AbbVie, fingernail psoriasis – which affects half of all psoriasis patients – involves pitting, deformation, thickening, discoloration, pain and separation of the nail from the nail bed. With the FDA approval, AbbVie’s Humira becomes the first biologic-based fingernail psoriasis treatment with data.
"Fingernail psoriasis is often difficult to treat, and we are pleased the FDA recognized the importance of these data so that more healthcare providers can make informed medical decisions when prescribing treatments for those living with psoriasis," Dr. John Medich, AbbVie's vice president for clinical development, said in a statement. "AbbVie's nearly 20 years of research in immunology provides us with in-depth knowledge of challenging diseases and allows us to identify ways to address them - it is our hope that the fingernail psoriasis data will support healthcare providers treating this difficult condition."
Humira was first approved by the FDA to treat psoriasis in 2008.










 Alerts Sign-up
Alerts Sign-up