CSL Behring's Haegarda drug, the only subcutaneous therapy for preventing hereditary angioedema (HAE) attacks in both adolescents and adults, was approved by the U.S. Food and Drug Administration.
“The FDA approval of Haegarda is a transformational milestone for the HAE community because it addresses the primary need of patients: to effectively prevent debilitating HAE attacks,” CSL Behring Chief Scientific Officer Dr. Andrew Cuthbertson, said in a statement. “CSL Behring has a long heritage of delivering on its promise to the HAE community. Thanks to our clinical trial participants, we’re proud to lead the community into the next era of treatment by offering the first and only subcutaneous preventive treatment option.”
According to CSL Behring, a Phase III study confirmed the safety and efficacy of Haegarda. In the study, a low dosage appeared to reduce the median number of HAE attacks by 95 percent.
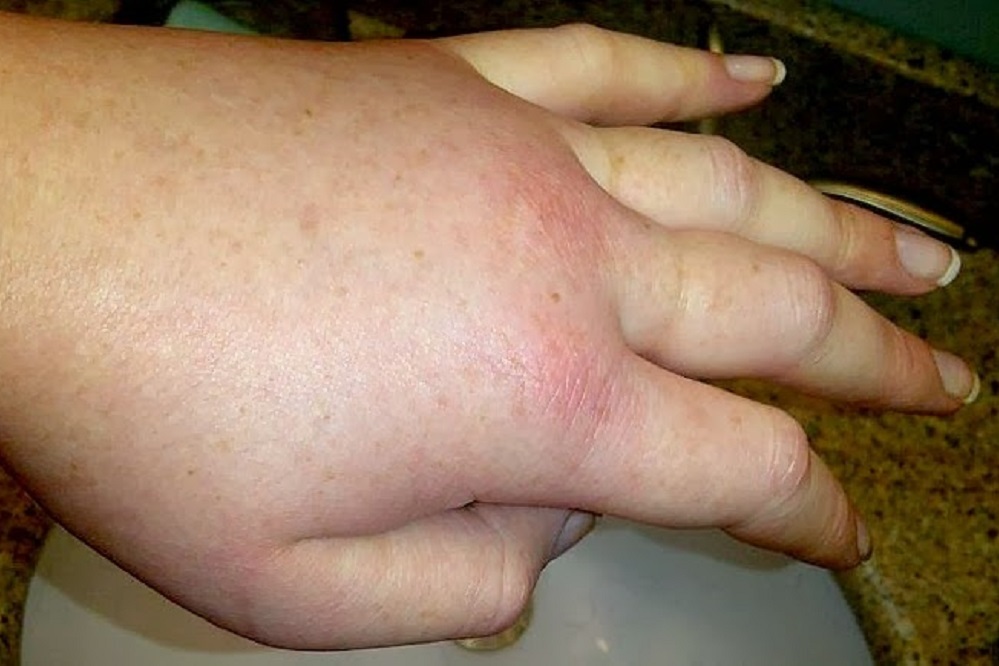
“HAE patients face painful, debilitating, and life-disrupting swelling episodes that are also life-threatening when the airway is involved,” Anthony Castaldo, president of the U.S. Hereditary Angioedema Association, said. “Patient care in the United States takes an important step forward with the approval of this subcutaneous treatment for preventing HAE attacks.”












 Alerts Sign-up
Alerts Sign-up