Cardiome Pharma Corp. partner SteadyMed Ltd. has submitted a New Drug Application for Trevyent with the U.S. Food and Drug Administration.
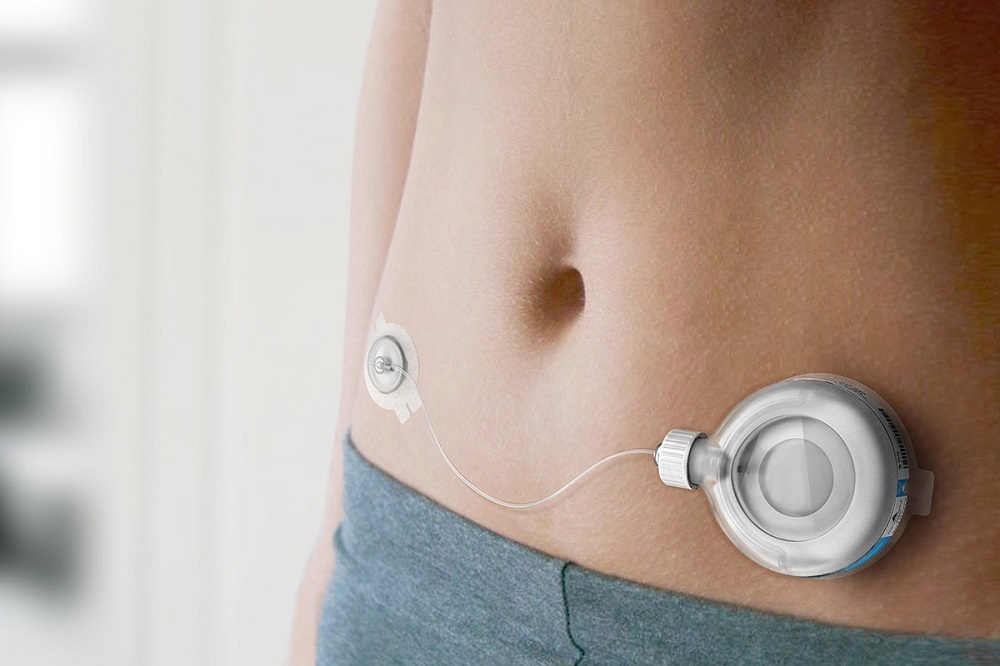
Trevyent is to be used with PatchPump for the delivery of treprostini, a treatment for pulmonary arterial hypertension (PAH). PAH is a high blood pressure classification that impacts the right side of the heart and the arteries that bring blood to the lungs. PAH can become life-threatening if too much time passes, as the pressure in arteries can become excessively high.
PatchPump is a disposable drug administration tool that is filled onsite with the manufacturer. It is expected that Trevyent will be approved by European Medicines Agency and Health Canada by the end of 2017.
"The NDA filing with the FDA is an important step in the development of Trevyent, and advances us closer to our goal of bringing what we believe is a better way to deliver treprostinil to patients around the world who are suffering from PAH," Cardiome's Chief Commercial Officer Hughes Sachot said.












 Alerts Sign-up
Alerts Sign-up