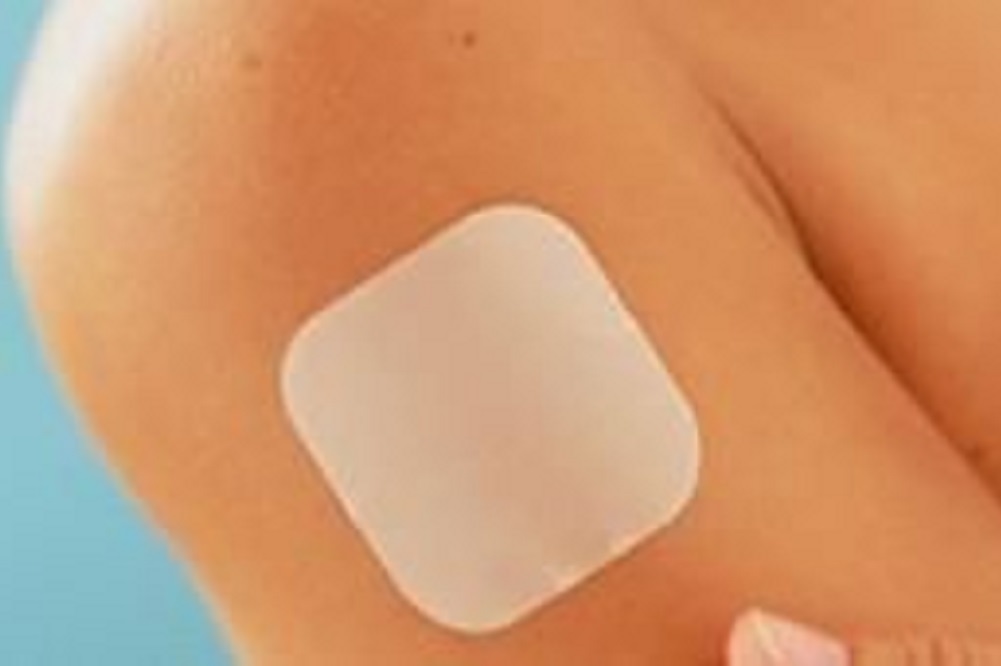
Progressive women's health care company Agile Therapeutics Inc. of Princeton, New Jersey has resubmitted its Twirla hormonal contraceptive patch to the U.S. Food and Drug Administration for a New Drug Application (NDA).
As the company’s lead product candidate, Twirla is an investigational low-dose transdermal patch. The FDA previously recommended in a February 2013 Complete Response Letter that Agile conduct an additional clinical trial before resubmitting the patch for consideration, as well as include more information on its manufacturing process.
The resubmitted NDA now contains efficacy and safety data based on the company’s new Phase 3 clinical trial as well as additional content.
"We … look forward to working with the FDA during the review process," Agile Chairman and CEO Al Altomari said. "Our achievement of this milestone reflects our commitment to changing the paradigm of available contraceptive treatment options for today's women and brings us one step closer to commercializing our low-dose contraceptive patch and offering an option to women seeking novel methods best suited to their needs and lifestyle.”
Altomari added that once the FDA processes the resubmission, Agile anticipates obtaining a review date. Twirla is based on Skinfusion, Agile’s proprietary transdermal patch technology.








 Alerts Sign-up
Alerts Sign-up