Sun Pharmaceutical Industries Ltd.'s Biologics License Application (BLA) for tildrakizumab has been accepted by the U.S. Food and Drug Administration.
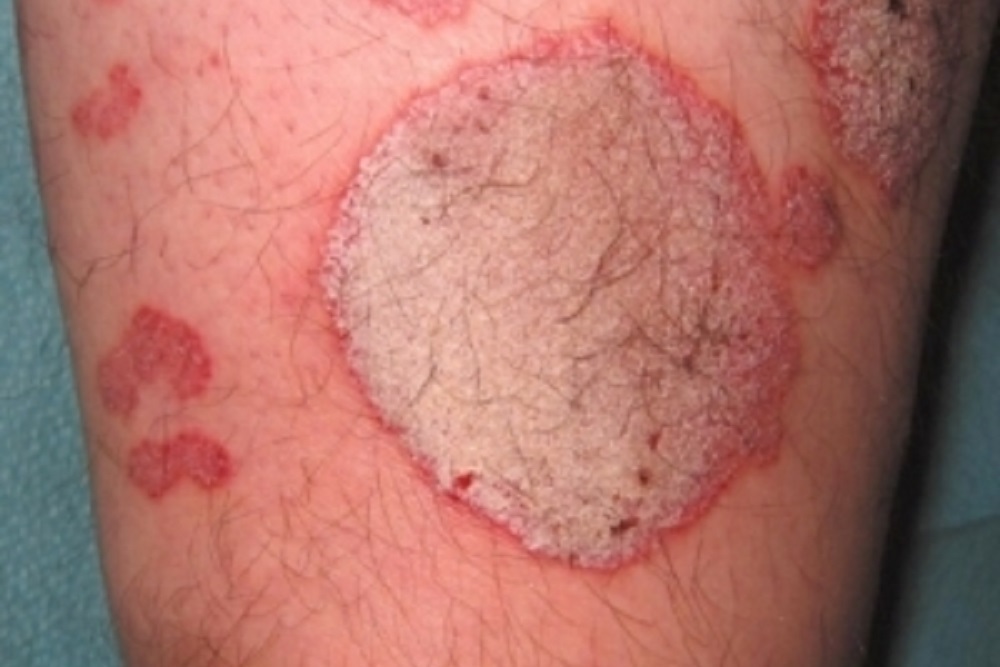
The drug treats psoriasis, a chronic immune disease that appears on the skin and affects roughly 7.5 million people in the United States and 125 million people globally. Tildrakizumab, an investigational humanized, anti-IL-23p19 monoclonal antibody, was created to control the pathogenic cells responsible for the psoriasis inflammatory process.
The company’s BLA filing for the drug comes after two Phase III trials conducted on 1,800 patients across more than 200 clinical sites. Sun Pharma presented data from these trials at the 2017 American Academy of Dermatology (AAD) Annual Meeting in March.
“At Sun Dermatology, we are committed to making a difference in the lives of patients and healthcare providers,” Abhay Gandhi, CEO for North America Business, said in a statement. “The acceptance of the regulatory filing by the U.S. FDA marks a significant milestone as we seek to advance for tildrakizumab as a potential new treatment option for people who continue to struggle everyday with the chronic nature of psoriasis.”








 Alerts Sign-up
Alerts Sign-up