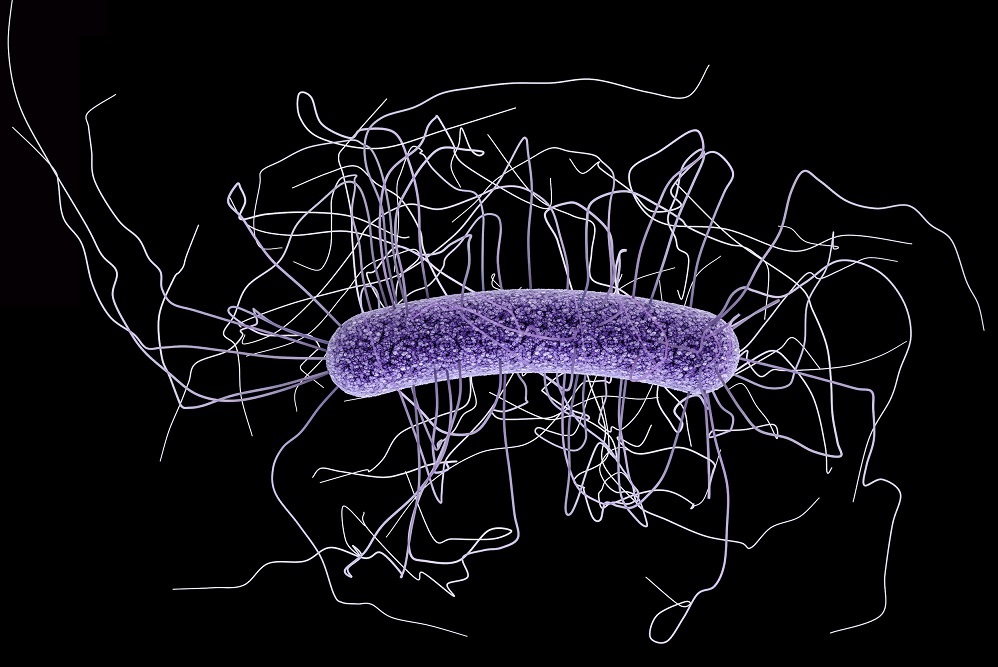
Luminex Corp.'s ARIES clostridium difficile (c. difficile) assay has been given U.S. Food and Drug Administration clearance.
"C. difficile infections have become more frequent, severe and difficult to manage in recent years, increasing our need for better tools to diagnose and treat these debilitating and life threatening infections,” Dr. Ray Widen, scientific director of esoteric testing and R&D at Tampa General Hospital, said in a statement.
According to the Centers for Disease Control (CDC), c. difficile is an urgent threat as it is now the most common microbial cause of health care-associated infections in U.S. hospitals. The CDC claims that almost 500,000 infections occurred in a single year in 2015.
"With this regulatory approval, we are further broadening our infectious diseases testing portfolio and adding to our rapidly growing menu of targeted assays and customized panels," Luminex President and CEO Homi Shamir said. “We are very pleased to offer a new solution to hospitals and molecular testing labs that can help improve their ability to test for these serious infections. From GI to respiratory, blood culture to women's health, we are making a true difference in the delivery of health care with the rapid number of high quality products we are bringing to market, including four new tests for the ARIES System already this year."








 Alerts Sign-up
Alerts Sign-up