Neovacs, a company focusing on immunotherapies that treat autoimmune diseases, has had its Investigation New Drug (IND) application for IFNalpha Kinoid cleared by the U.S. Food and Drug Administration (FDA).
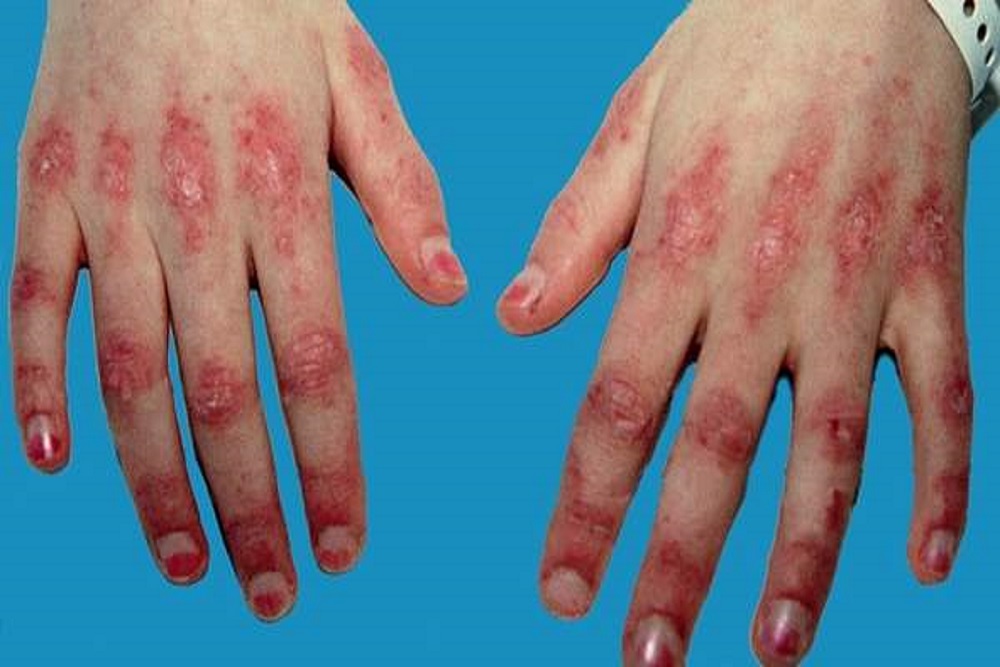
IFNalpha Kinoid treats dermatomyositis; the FDA’s clearance allows the drug to be studied in a Phase IIa clinical trial in the United States. The company noted the same clinical trial is already underway in Europe in 30 adult patients.
The trial will examine the immunogenicity, tolerability and biological and clinical efficacy of IFNalpha Kinoid in this new indication.
“FDA clearance of a new IND application is an important milestone for the development of our IFNalpha Kinoid technology,” Neovacs CEO Miguel Sieler said in a statement. “The data obtained in lupus with IFNalpha Kinoid have been positively evaluated by FDA, which supports the application of our vaccine in dermatomyositis. Therefore, we are focused on advancing IFNalpha Kinoid through the clinic, as expeditiously as possible, in the context of an orphan disease with a high unmet medical need."








 Alerts Sign-up
Alerts Sign-up