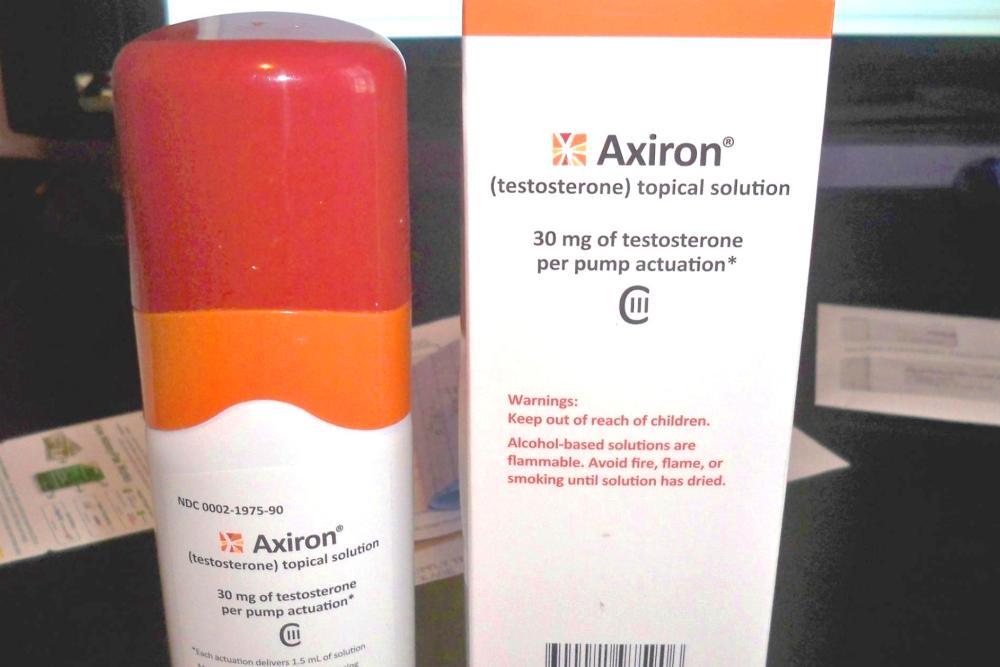
Jerusalem-based Teva Pharmaceutical Industries Ltd. recently began marketing generic Axiron (testosterone) topical solution CIII, 30 mg/1.5 mL, in the United States for treating adult males who are experiencing low testosterone levels due to medical reasons.
“We are pleased with the result in the district court, which has helped Teva add yet another product to our industry-leading generic portfolio, providing savings to our customers and to patients,” Andy Boyer, Teva’s president and CEO for global generic medicines in North America, said. “Teva continues to lead the industry in bringing new generic products to the U.S. market.”
Axiron addresses primary hypogonadism, or testicular failure, that ensues because of a variety of medical conditions. Patients typically experience low serum testosterone concentrations but higher than average gonadotropin (FSH, LH) levels. It is a topical solution dispensed with the help of an underarm applicator used with a metered dose pump.
Teva provided information regarding risks, contraindications, and possible adverse reactions including skin sensitivity, elevated hemocrit levels, headache, diarrhea or vomiting, as well as increased serum prostate specific antigen.
Teva distributes almost 600 different generic medications. One out of every six generic prescriptions nationwide is filled with a product made by Teva, according to company spokespersons.







 Alerts Sign-up
Alerts Sign-up