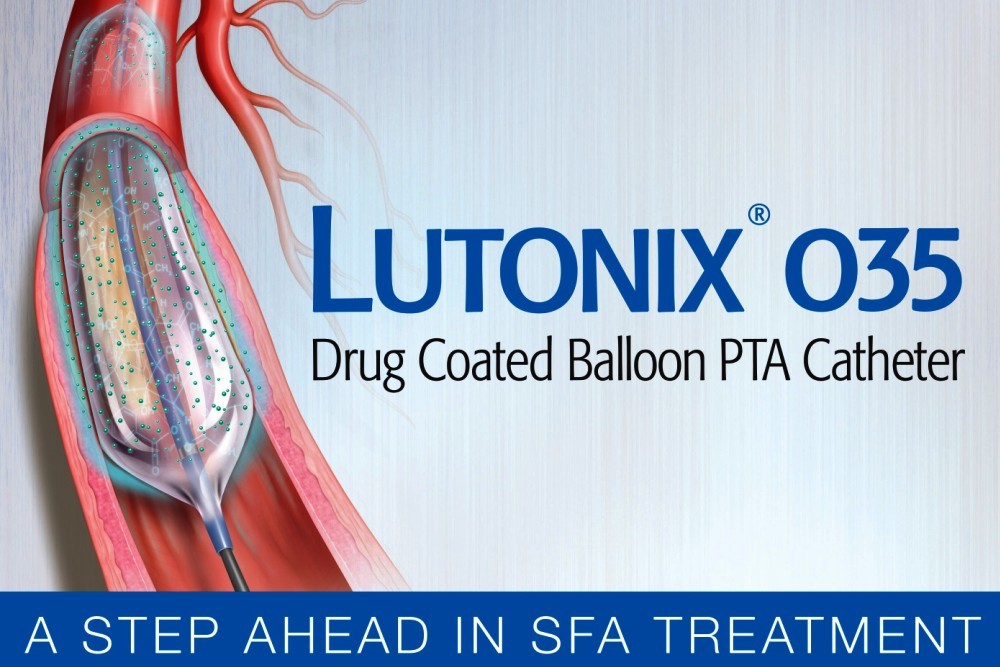
New Jersey-based surgical product producer and marketer C. R. Bard recently obtained premarket approval from the U.S. Food and Drug Administration for its Lutonix 035 drug-coated balloon (DCB) PTA catheter.
The treatment is designed for use in conjunction with kidney dialysis, which often depends on the successful connection of an artery to a vein, known as an AV (arteriovenous) fistula.
As the first product of its kind indicated for treatment of end stage renal disease in individuals experiencing stenotic lesions in AV fistulae, Lutonix also obtained previous agency approval for other artery disorders.
This new approval followed a clinical trial indicating 71.4 percent effectiveness (measured at 210 days) as well as 31.3 percent fewer re-intervention days recorded (at 217 days).
“This approval offers a new treatment option for patients suffering from end-stage renal disease,” C. R. Bard Chairman and CEO Timothy Ring said. “In line with our continued commitment to deliver products that improve patient care, we are proud to extend the benefits of the Lutonix 035 DCB Catheter to help preserve treatment options for U.S. patients.”
Over 2 million patients are undergoing hemodialysis, C.R. Bard officials said. Principal study investigator Dr. Scott Trerotola noted that renal patients — many of whom spend large amounts of time in treatment — can benefit from Lutonix’s potential to ameliorate treatment.
“The Lutonix 035 DCB Catheter provides another option for physicians,” Trerotola, who serves as associate chair and chief of interventional radiology at the University of Pennsylvania School of Medicine, said. “It’s intended to offer patients with end-stage renal disease fewer interruptions in treatment and less time undergoing access maintenance, potentially leading to improved patient satisfaction and quality of life.”








 Alerts Sign-up
Alerts Sign-up