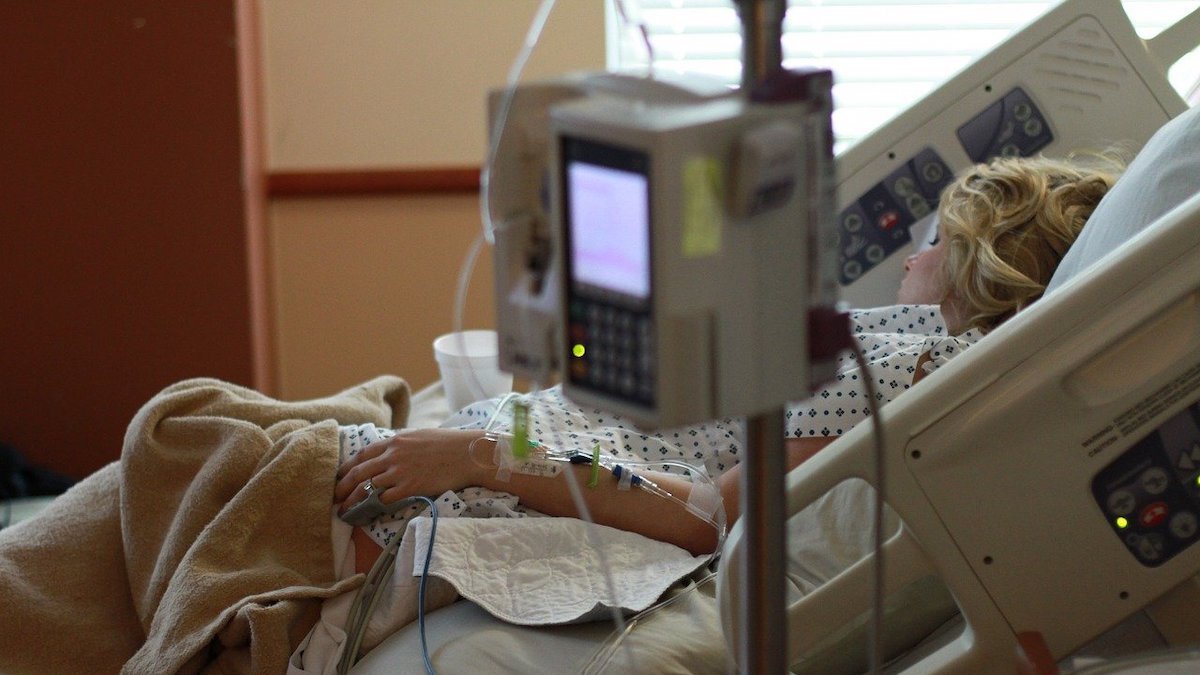
The majority receiving COVID-19 experimental treatments are those patients hospitalized with the coronavirus, Cedars-Sinai Hospital Epidemiology Director Jonathan Grein said in a recent news release.
It's typical to provide experimental treatments for new diseases to those suffering most from the new disease, Grein said in a Cedars-Sinai news release issued July 20.
"Patients who are sicker and in the hospital usually have a more advanced illness, and you want to do everything you can to find something that will help them," Grein said. "Hospitalized patients also can be monitored closely for their reaction to a drug."
The U.S. Food and Drug Administration has already approved many of those COVID-19 experimental treatments, which makes those treatments easier to deploy, according to the news release.
Those experimental treatments include anti-viral drugs, such as treatments for influenza, HIV and different types of hepatitis, according to the news release.
Experimental anti-inflammatory treatments, such as steroids and IL-6 inhibitors, which tamps down a patient's immune system’s response and are more likely to be used in late state COVID-19, according to the news release. Those mediations also carry the potential of making a patient more vulnerable to COVID-19.
"We need to be judicious about how and when we use these," Grein said in the news release.
Cedars-Sinai, a non-profit academic healthcare organization headquartered in Los Angeles with locations throughout southern California, is known worldwide for its pioneering medical research and education programs.
Though the pandemic has been known to be active in the U.S. only for months, many new experimental therapies are now in clinical trials and COVID-19 research is happening "at breakneck speed," the news release said.
"Our understanding of potential treatment options is changing rapidly because there has been a tremendous focus in the scientific community on identifying options from a variety of angles," Grein said in the article. "It's been great to see everybody focused on trying to find therapeutic options that work. But I'd urge the public to be cautious when interpreting headlines about new developments because we need to ensure that the science is sound before recommending anything definitively."










 Alerts Sign-up
Alerts Sign-up