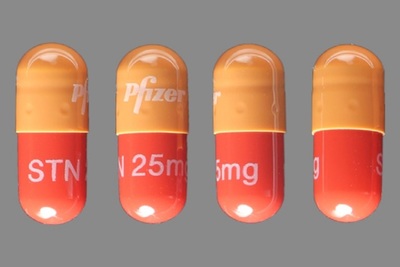
PFIZER: Announces Update on European Marketing Authorization Application for SUTENT® (sunitinib) in Adult Patients at High Risk of Recurrent Renal Cell Carcinoma
Pfizer Inc. (NYSE:PFE) announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended against expanding use of SUTENT® (sunitinib) to include the adjuvant treatment of adult patients at a high risk of recurrent renal cell carcinoma (RCC) following nephrectomy (surgical removal of the cancerous kidney). Read More »























 Alerts Sign-up
Alerts Sign-up