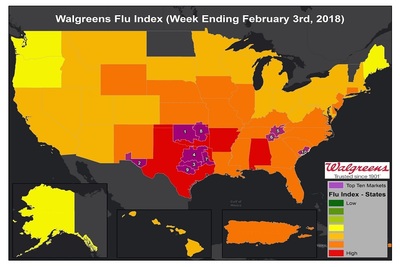
WALGREENS INC.: Tennessee and North Carolina Markets Top List of Flu Activity Gains
The Walgreens Flu Index™ is a weekly report developed to provide state- and market-specific information regarding flu activity, and ranks those states and markets experiencing the highest incidences of influenza across the country. Read More »

















 Alerts Sign-up
Alerts Sign-up