Cidara to present CD101 data at ACCP and IDWeek meetings
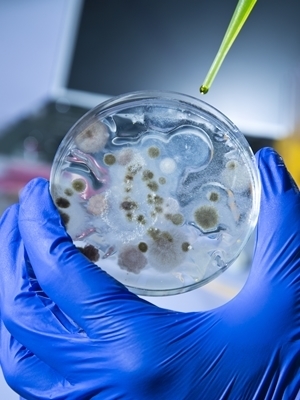
Cidara Therapeutics' data from its CD101 preclinical and clinical trials was presented at the 2016 American College of Clinical Pharmacy annual meeting and at the IDWeek 2016 meeting last month. Read More »




.jpg)




.jpg)















 Alerts Sign-up
Alerts Sign-up