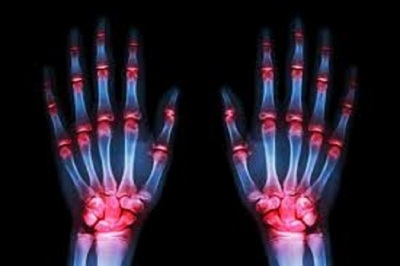
Pfizer's Xeljanz receives positive opinion from European committee
Pfizer Inc. recently announced that its Xeljanz drug for treating moderate to severe active rheumatoid arthritis (RA) has received a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA). Read More »


















 Alerts Sign-up
Alerts Sign-up