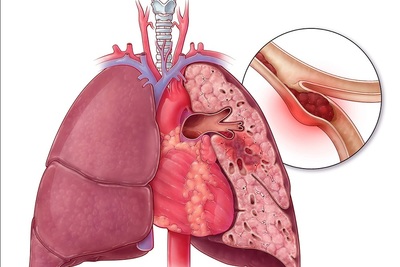
Portola's Bevyxxa approved for patients at risk of thromboembolic complication
Portola Pharmaceuticals recently announced that its Bevyxxa drug was approved by the U.S. Food and Drug Administration (FDA) for treating adult patients hospitalized for an acute medical illness who are at risk for thromboembolic complication. Read More »













 Alerts Sign-up
Alerts Sign-up