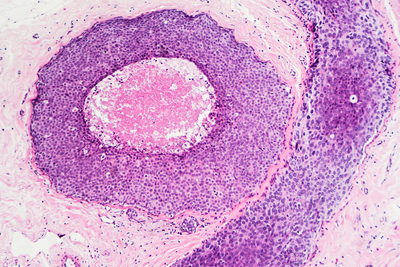
FDA grants priority review to carcinoma drug
EMD Serono, the biopharmaceutical business of Merck KGaA and Pfizer Inc., recently announced that the Biologics License Application (BLA) for their avelumab drug was accepted for Priority Review by the U.S. Food and Drug Administration (FDA). Read More »


.jpg)











.jpg)
.jpg)
.jpg)




 Alerts Sign-up
Alerts Sign-up