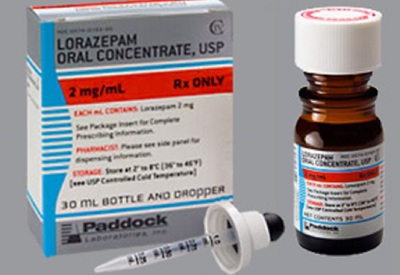
Amneal Pharmaceuticals recalls misprinted Lorazepam dispensers
Amneal Pharmaceuticals, based in Bridgewater, New Jersey, has voluntarily recalled 13 lots of Lorazepam Oral Concentrate, USP 2mg/mL, Lorazepam Oral Concentrate, USP 2mg/mL — a product for which the accompanying dropper dispenser may be erroneously labeled. Read More »





















 Alerts Sign-up
Alerts Sign-up