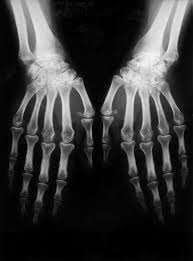
Incyte Corp. and Eli Lilly and Company recently announced that its investigational medicine baricitinib proved superior to the placebo that was used in the Phase 3 trial study for rheumatoid arthritis (RA).
The Phase 3 study involved over 3,000 subjects suffering from different stages of rheumatoid arthritis. The patients received one or two doses of either the baricitinib or the placebo treatments.
The study was specifically designed to find treatments for patients with moderate to severe rheumatoid arthritis. These patients previously showed such a low response or such a high intolerance of the traditional conventional disease-modifying antirheumatic drug that new treatment needed to be developed. Included in the study were 684 such patients.
"We are encouraged that baricitinib demonstrated statistically significant improvement in rheumatoid arthritis disease activity compared to placebo, now in a second pivotal study," Lilly senior vice president and president of Lilly Bio-Medicines
David Ricks said.
After receiving the treatment for 12 weeks, the subjects showed an enhanced ACR20 response rate that outshone the placebo.
"Despite treatment advances, many people with RA continue to experience active disease, including pain, joint stiffness, disability and progressive joint damage," Incyte Corp. chief drug development and medical officer Rich Levy said. "These results suggest that baricitinib could provide an additional treatment option for patients who are not responding to conventional drugs."









 Alerts Sign-up
Alerts Sign-up