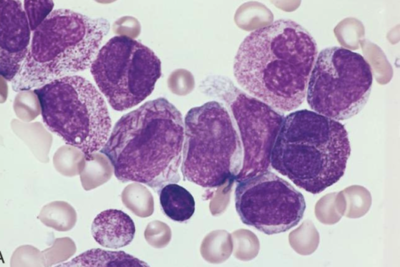
FDA grants Breakthrough Therapy Designation to lymphoma drug
AstraZeneca and Acerta Pharma's treatment for patients with mantle cell lymphoma who have received at least one prior therapy was granted Breakthrough Therapy Designation by the U.S. Food and Drug Administration. Read More »

















 Alerts Sign-up
Alerts Sign-up