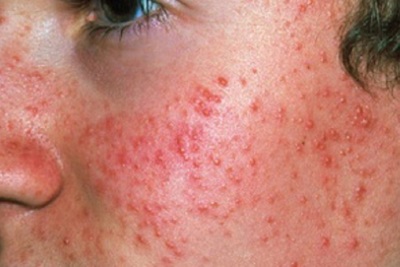
BioPharm shares pre-clinical data for BPX-01 topical gel
BioPharm X Corp., a specialty pharmaceutical company focused on the dermatology market, recently shared early clinical and pre-clinical data for BPX-01 topical gel at the 2017 American Academy of Dermatology (AAD) Annual Meeting. Read More »



















 Alerts Sign-up
Alerts Sign-up